Philips CPAP recall: Do you want cancer or a heart attack?
A look at their proactive communication measures and business ethics
The CPAP industry is notoriously known for being an information-gated industry. In 2018, Mark Watkins, the creator of SleepyHead, a software that gives you access to your sleep data, had this to say.
“I became increasingly disgusted at how the CPAP industry is using and abusing people”
Does this comment still ring true a few years later? Recently, Phillips Respironics issued a voluntary recall for ~4 million CPAP machines and other breathing devices due to health risks. The foam used to make these machines quieter was found to degrade over time, producing toxic chemicals. If ingested, this could cause headaches, respiratory issues and cancer causing effects.
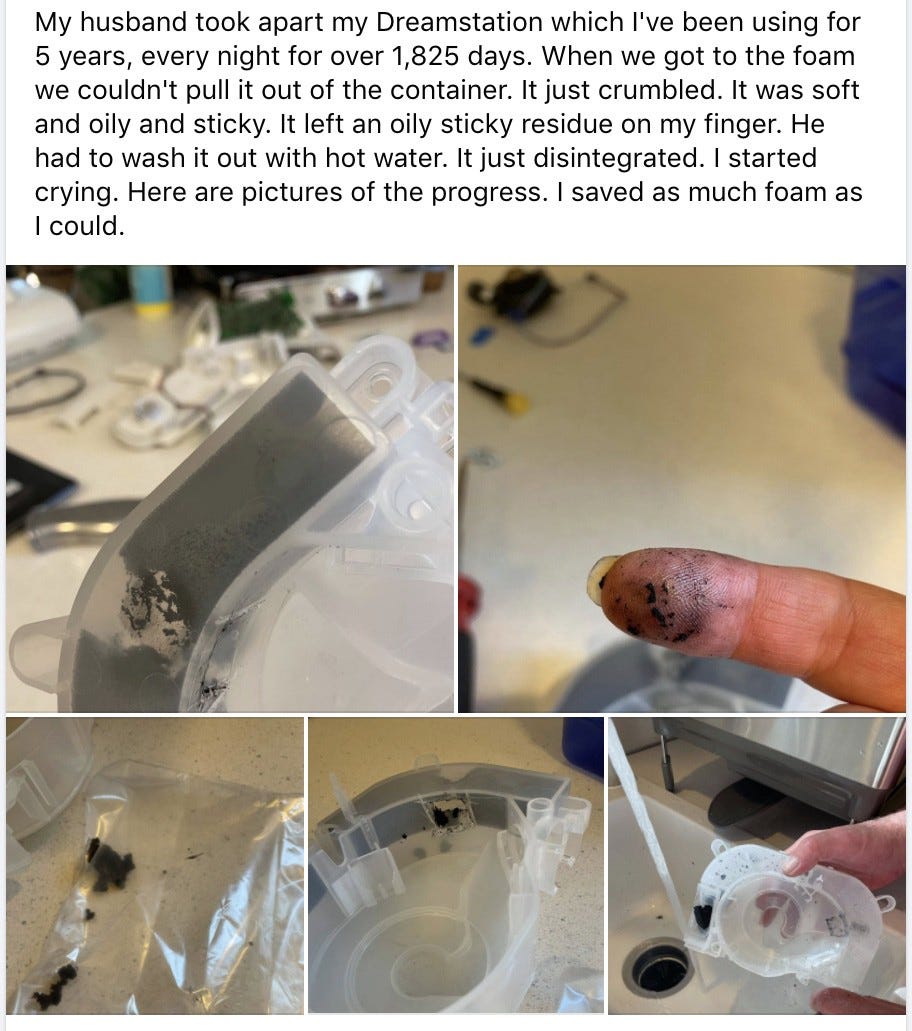
Here’s what the degraded foam looks like.
In an official notice, Philips recommended stopping use of these machines where possible. However, not everyone has the luxury of alternative treatments. Buying a new machine can range from $1000–1500. Instead, they ask you to consider if using the device outweighs the risks of sleeping without it. Essentially, do you want to increase your chances of cancer or cardiovascular disease? Either way, it is impossible to sleep with peace of mind.
Philip’s Response, a Proactive Approach?
Philip’s official update (April 26th, 2021) states they are taking a proactive approach.
There is nothing we take more seriously than providing patients with high quality products that are safe and reliable. If an issue arises, we are proactive in communicating and addressing it as we work tirelessly towards a resolution.
Their CEO, Frans van Houten also further reassures the public a few months later with this:
We deeply regret any concern and inconvenience that patients using the affected devices will experience because of the proactive measures we are announcing today to ensure patient safety . . . Patient safety is at the heart of everything we do at Philips
So how proactive have they been with communicating this to their patients? Let’s take a look.
Across all their official social media accounts, there has been zero mention of the product recall or associated health risks. At least one post would even classify as the bare minimum.
For those using the devices, it is often advised to download their mobile app called DreamMapper which lets you see your therapy progress. The app sends push notifications for reminders and advice on how to improve your therapy.
You would imagine this would be the most direct and beneficial way of communicating the recall to affected devices. Nick from CPAP Review, an educational channel on CPAP devices and sleep apnea posted a poll asking this very question.
Out of over 700 votes, only 5% percent received a notification.
For those that did receive a notification, it appears Philips are only contacting individuals that have already stopped using the device.
On June 14th, information was provided to clinicians detailing why products were recalled. Aside from foam degradation, they have also identified the risk of breathing in volatile organic compounds (VOC).
Potential Harm: During initial or subsequent operation of the device, a patient may be exposed to VOCs. . . . toxicological risk assessment indicates that VOCs levels exceed a safe exposure threshold.
Source: Page 5 of Clinical information for physicians
From day one, you are already exposed to chemicals outside the safety threshold.
Ethics
In 2016, Philips published an article titled “Why placing ethics over profits pays off”.
As I put it to my organization: the only business that we do is ethical business
This is not the first time Philips has been involved with something ethically questionable. In 2019, they were investigated by the FBI for suspected corruption of medical equipment sales. More recently, they were investigated for charging the US government four times the price for 43,000 ventilators.
So the question is, how long have they known about the risks? The recall notice was published April 26th, 2021. The release date of the DreamStation 2 was also released in April 2021. Coincidentally, their newest device is unaffected, unlike all its predecessors spanning over the last decade.
If the foam was defective, it makes sense to wonder where it’s being made. When asked this in their earnings call conference, Frans had this to say.
Okay. And Frans, you didn’t answer the question about who the foam manufacturer is? Can you highlight that, please? Could you mention that?
Frans van Houten Yes. I realized that I didn’t answer that. So I am not going to answer that.
Source:
I guess we’ll never know.